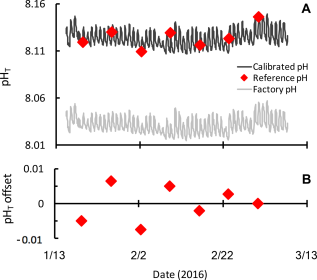
SeaFET pH time series showing the difference between the factory calibration (grey) and calibration using multiple discrete pH samples collected during a single deployment (black, A). Discrete samples (red) were analyzed using the spectrophotometric pH method with m-cresol indicator dye. Discrete samples show a narrow offset (<0.01 units pHT) from the final calibrated time series (B). These data are a subset of a longer pH time series from the coastal Mediterranean Sea (Kapsenberg et al. 2017a) and a courtesy of Samir Alliouane and Jean-Pierre Gattuso.
Workshop participants in front of the Bay of Villefranche, France.
To expand expertise on Durafet-based ocean pH sensors, Samir Alliouane and I ran a two-day workshop on “Best practices for Durafet-based ocean and laboratory pH time-series” at the Villefranche Oceanographic Laboratory in France. The aim of the workshop was to provide participants with a technological overview and the most up-to-date scientific recommendations and data processing tools. Emphasis was placed on understanding the principles of calibration using discrete reference samples, data interpretation, and reporting. Seventeen participants represented nine different countries and ranged from recent PhD graduates to senior research scientists. Most participants were still in the planning stages or had recently started using Durafet-based ocean sensors. Participants had access to a prototype and commercial SeaFET pH sensor as well as Durafets configured for laboratory experiments using commercial analyzers. The workshop highlighted key aspects of Durafet-based sensors that some novice users had previously not considered. The most important aspect was the necessity of acquiring independent pH measurements on discrete seawater samples for calibration and quality control (Figure 1). Ideally, discrete samples are collected in-situ at the deployment site while the sensor is operating. The sampling strategy for this depends on feasibility of frequent site visits, duration of the deployment period, and onset of biofouling. The latter is site-specific and helpful to know before designing a deployment strategy. Discrete samples are analyzed for pH and used to post-calibrate the pH time series coming from the sensor (Bresnahan et al. 2014). As with any instrument, the data are only as accurate as the calibration, so it remains important for users to quantify the level of uncertainty associated with their discrete pH measurements. For sensor packages dealing with raw voltage data, such as the SeaFET™ and SeapHOx™, we introduced new R-functions, which were translated from MATLAB (Bresnahan et al. 2014). These R functions are available in the updated ‘seacarb’ R-package (Gattuso et al. 2017). Use of Durafet sensors in laboratory-based experiments requires different considerations for seawater pH calibration; these methods are outlined in an upcoming publication (Kapsenberg et al. 2017b).
At the conclusion of the workshop, participants indicated a new awareness about the extent of data post-processing required for Durafet-based time series. Others indicated that they would reexamine their initial deployment strategy to maximize the return of high-quality data and minimize effects from common problems such as biofouling.
This workshop established a new international network of marine scientists studying ocean pH dynamics. We hope that this training will lead to high-quality pH observations and advance knowledge on ocean acidification across different marine ecosystems.
Acknowledgments
This workshop was made possible by the National Science Foundation postdoctoral research fellowship awarded to L. Kapsenberg (OCE-1521597).