July 17, 2017

A tiny sea snail, sometimes called a sea butterfly because of how it flutters about traveling the ocean currents, is part of the diet for such valuable fish as salmon and cod off the U.S West Coast.
A new study models the journey of this delicate plankton from offshore to nearshore waters, describing how changing ocean chemistry along this journey affects their condition.
During its travels the sea snail, or pteropod, encounters increasingly harmful conditions as it moves into nearshore waters. These waters are more corrosive to the organism due to higher levels of carbon dioxide coming from upwelled deep water. In recent years these upwelled waters have grown increasingly corrosive as a result of ocean acidification (OA) caused by the uptake of rising levels of atmospheric CO2.
Predicting effects of ocean acidification
To predict the future effects of increasing ocean acidification on the sea snail, NOAA and partner scientists developed a high resolution numerical model to track its movements in the California Current Ecosystem and measure how much exposure to corrosive conditions the tiny snail endures over its journey and how quickly these conditions disrupt it.
The results published recently in Nature Scientific Reports show that the pteropod can be rapidly affected as it moves into more corrosive nearshore waters.
“Our model shows that within days to weeks, pteropods transported by currents from the open-ocean to more corrosive nearshore waters have difficulty building and repairing their shells,” said Nina Bednarsek, Ph.D., a research scientist from the Southern California Coastal Water Research Project in Costa Mesa, California, and lead author of the study. “The model shows pteropods are less likely to survive after five to six weeks of exposure to OA conditions.”
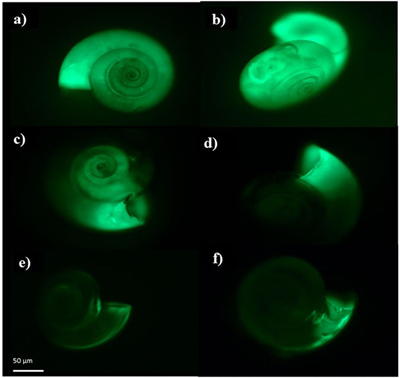
Shell building declines This series of pteropod images shows that as pteropods are exposed to increased ocean acidification in nature, their ability to build shells declines. Shell-building activity is indicated by the strength of the fluorescent green glow which is brightest when more calcium carbonate mineral is being incorporated by the pteropods. When the concentration of calcuim carbonate declines due to ocean acidification, the image shows a dimming green, indicating little or no shell building is occurring. Credit: N. Bednarsek
To develop the model, scientists coupled ocean chemistry observations taken during a 2013 NOAA research cruise along the West Coast, and information from laboratory experiments testing how pteropods build and repair their shells when exposed to increased levels of acidification in seawater.
“The new model lays the groundwork for understanding the effects on the pteropod and may help us understand how ocean acidification affects other microscopic organisms carried by ocean currents,” said Richard Feely, NOAA oceanographer and a study co-author.
Over the last 250 years, the global ocean has absorbed an estimated 550 billion tons of carbon dioxide from emissions released into the atmosphere from burning wood or fossil fuels, land-use changes and cement production, according to past research. The California Current Ecosystem along the West Coast is particularly vulnerable to ocean acidification because it not only absorbs carbon dioxide from the atmosphere but is also bathed by seasonal upwelling of carbon-dioxide rich waters from the ocean interior.
Original article: http://research.noaa.gov/
For more information, please contact Monica Allen, director of public affairs for NOAA Research, at 301-734-1123 or by email at monica.allen@noaa.gov